Which of the Following Has No Net Dipole Moment
Which of the below molecules has no net dipole moment. 101Which of the following has no net dipole momentN2O NF3 H2Se TeO3 CH3Cl.

Answered Which Of The Following Has No Net Bartleby
Thus a molecule such as H 2 O has a net dipole moment.
. Which of the following molecules is polar. We expect the concentration of negative. Among all the given options TeO 3 has no net dipole moment because the central atom has three bonded atoms one bond being a double bond and has no lone pairs and the geometry is trigonal planar with symmetrical distribution of dipoles resulting in dipole cancellations and has no net dipole moment.
C H C l 3 has net dipole moment as the bond dipoles do not cancel each other. Which of the following does not have a molecular dipole moment. In contrast the H 2 O molecule is not linear part b in Figure 228.
First week only 499. D H 2 O. Predict the ideal bond angles in FNO using the molecular shape given.
Therefore CO2 has no dipole moment. Correct Answer - BCD cis form have some net dipole moment. Start your trial now.
Larger hydrogen-bond forces for H2Te. PH3 HBr CH3OH CH3CH3 CH3CH3 The normal boiling point for H2Te is higher than the normal boiling point for H2Se. As a result the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge.
1-Which of the following has no net dipole moment. 95 130 ratings play-rounded-fill. Only D has a net dipole because A is tetrahedral and B is linear causing theindividual bond dipoles to cancel outO O O O O O.
Which of the following molecules has a net dipole moment. It is bent in three-dimensional space so the dipole moments do not cancel each other. And whya CH4 b CHBr3 c F2 dCBr4 e CO23-.
The polarization of the 3 B-Cl bonds exactly. Which of the following alkanes has no net dipole moment. This can be explained by larger dispersion forces for H2Te.
For H 2S the bonds are both polarized but H 2S is a bent molecule not linear so the polarizations do not cancel and H 2S has a net dipole moment. Whereas other molecules such as C H 4 C O 2 and C C l 4 have zero dipole moment as the bond dipoles completely cancel each other. Thus CCl4 has no dipole moment.
A BF3 B NCl3 C H2Se D CH3Cl. The F-S-F bond angles in SF6 are. Solution for Which of the following has no net dipole moment.
2 CCIA 3 BeF24 SO Open in App. This browser does not support the video element. The structure of compounds given in options are as follows.
Compounds A B and D have polar bonds. Answered Aug 28 2021 by MehulKumar 187k points selected Aug 30 2021 by Ritwik. Which of the following has a net dipole moment.
So according to this the correct option is d. For BCl3 the geometry is an equilateral triangle of Cl atoms with the boron atom in the center of the triangle. A SCl2 B H2O C CF4 D BrCl.
Andwhya H2O bNF3 cH2Se dTeO3 e CH3Cl2-. A 90 and 180 b. Compounds C and E are non-polar.
A molecule which has a symmetrical geometry will have no dipole moment as the magnitude of all the bond moments cancel each other. Which of the following molecule has net dipole moment. O CH CI NF3 N20 O TeO3 H2Se O O O.
A BeCl2 B CCl4 C CO2 D SF2. Which of the following molecules does not have a dipole moment. Larger dipole-dipole forces for H2Te.
Which of the following compounds have net dipole momentA CBr4B CO2 C CH4D H2O E C2H4Ans. Which of the following has no net dipole moment. Weve got the study and writing resources you need for your assignments.

Solved Which Of The Following Has No Net Dipole Moment A Chegg Com

Solved 1 Which Of The Following Has No Net Dipole Moment O Chegg Com

Which Of The Following Alkanes Has No Net Dipole Moment Youtube

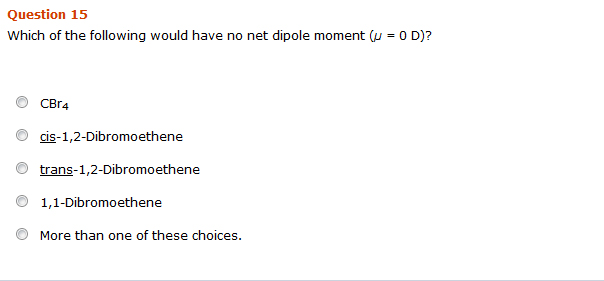
Solved Which Of The Following Would Have No Net Dipole Chegg Com
No comments for "Which of the Following Has No Net Dipole Moment"
Post a Comment